Oxidation is a fundamental process in which elements combine with oxygen, resulting in the displacement of electrons from the orbit of an atom’s nucleus. This leads to an increase in positive or proton valence, a phenomenon that can have both beneficial and detrimental effects on the body. On one hand, oxidation can assist in alkalization, promoting a balanced pH environment. On the other hand, it can give rise to free radicals that wreak havoc on cells, particularly in the presence of inflammation and acidosis. Acidosis occurs when there is an excess of acid in the body, leading to the formation of superoxide radicals when oxygen compounds are not fully broken down or utilized due to the inflammatory condition. This further contributes to cellular breakdown and damage.
The breakdown and utilization of oxygen compounds are hindered when our antioxidant levels are low or when our immune system is hypoactive (underactive). When proper ionization or neutralization fails to occur, these free radicals amalgamate, exacerbating tissue damage. This realization underscores the significant demand for foods rich in antioxidants. Antioxidants possess the unique ability to bond with free radicals, neutralizing their detrimental effects.
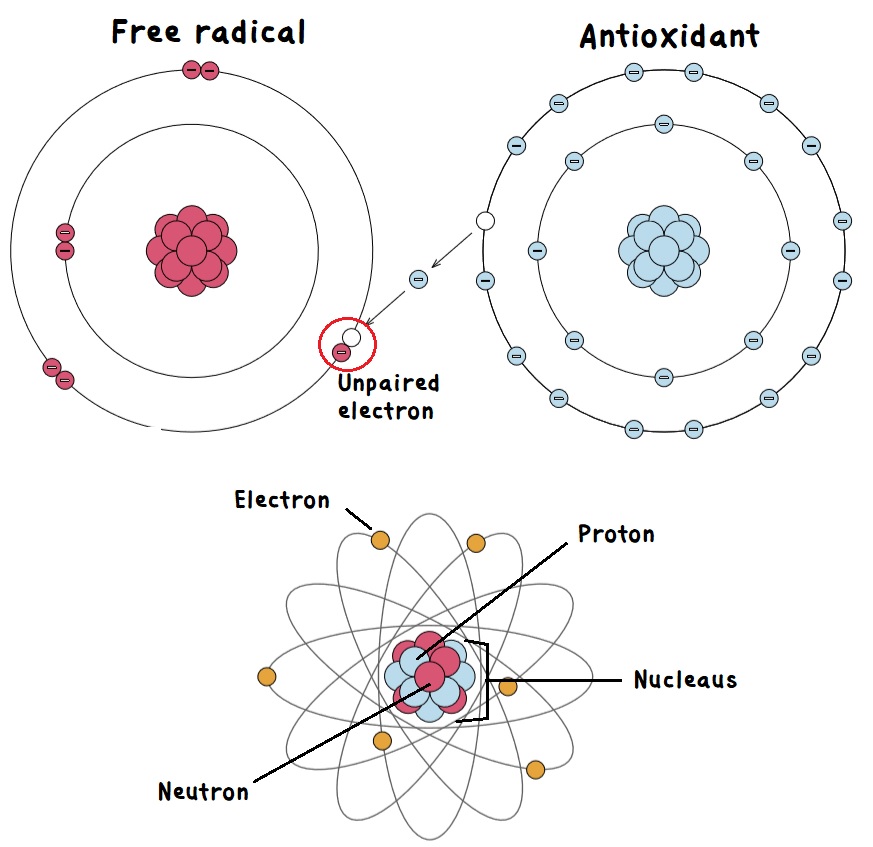
Oxidation also leads to ionization, a process through which elements or compounds are transformed into simple ions. Elements can initially carry a negative charge (alkaline), resulting in a cationic reaction. However, through ionization, the same element can become positively charged (acidic), leading to an anionic reaction. Consider the example of calcium, an alkaline element derived from plants. In conjunction with other elements like magnesium, sodium, and potassium, calcium alkalizes the body’s fluids. These elements, known as electrolytes, play a vital role in maintaining the body’s balance. However, once these elements enter the bloodstream, they can become ionized and magnetically attracted to other elements. This interaction can give rise to an acidic complex, turning them anionic. For instance, the combination of calcium and phosphate results in the formation of calcium phosphate, essential for building the skeletal system. Yet, free radicals like oxalates can also form calcium oxalate stones, leading to inflammation and tissue damage.
Water acts as a catalyst in the process of oxidation. When water interacts with metals, it facilitates rust formation. Similarly, in our blood serum, oxidation gives rise to electrolytes, which act as conductors of energy. Ionization generates positive ions (calcium, magnesium, potassium, sodium) and negative ions (chloride, sulfates, phosphates, carbonates). Ions serve as catalysts, akin to enzymes, triggering actions and reactions, building and breaking down. Alkalies foster anabolism, facilitating growth, rebuilding, and the creation of various aspects of life. Acids, conversely, promote catabolism, breaking down and destroying elements of nature. These two forces are intrinsically interconnected, with alkalinity facilitating movement, cleansing, and dispersion within the body, while acidity promotes coagulation, stagnation, and mass formation. Acidosis, characterized by excessive acidity, gives rise to malnutrition, inflammation, stone formation, pain, electrolyte depletion (dehydration), swelling, convulsions, and ultimately, death. Nature seemingly favors alkaline solutions.

We must always bear in mind that alkalies serve to neutralize acids, holding the key to regeneration and optimal health.
